Good news, friends. The paper that I mentioned in the last newsletter that was in peer review purgatory was recently accepted. It’s now online and available for free download from the journal via this link until April 03, 2022. Get it while it’s hot.
As a reminder here are some of the key deets:
This paper is called Leveraging DOM UV absorbance and fluorescence to accurately predict and monitor short-chain PFAS removal by fixed-bed carbon adsorbers. Let me work backwards to unpack the title:
“Carbon absorbers” - a generic way to refer to biochar or granular activated carbon (GAC). The study was done with biochar, but the results are applicable in a GAC context as well.
“Fixed-bed” - engineering-speak for granular media, in a tank, that stays stationary while you flow liquid (or gas) over it. The fluid flows, the media doesn’t.
“Short-chain PFAS” - a subset of per-/poly-fluoroalkyl substances (PFAS) whose carbon backbone is shorter than the more well known “legacy” PFAS pollutants like perfluorooctanesulfonatic acid (PFOS) and perfluorooctanoic acid (PFOA). Short-chain PFAS are smaller, more water soluble, less well characterized in terms of occurrence, environmental fate, and toxicity, and more difficult to remove by adsorption than larger PFAS molecules. Our sentinel PFAS PFBS and GenX are classified as short-chain and were included in the study.
“Monitor…” Challenge #1 addressed in the paper - monitoring PFAS removal in carbon adorer treatment systems. Measuring PFAS in water (at their typical ng/L levels) is complicated and expensive. Only a few labs around the world have the analytical capabilities and expertise to do so and it costs an arm and a leg. Spectroscopic water quality assessment methods such as measuring ultraviolet (UV) absorbance and fluorescence are comparatively very inexpensive. These methods don’t measure PFAS, but are sensitive to different components of background dissolved organic matter (DOM) - which causes fouling of the adsorbent and therefore relates to how well the adsorbent can remove target PFAS compounds. In the paper we show how monitoring UV absorbance and fluorescence can be used as proxy measures to assess carbon adsorber performance for PFAS removal.
“Predict…” Challenge #2 addressed in the paper - rapid laboratory bench top experiments are unreliable for accurately simulating removal of PFAS (and other trace pollutants) by full-sized real-world treatment systems. The reasons is that small lab bench experiments don’t adequately capture the effects of DOM fouling as they occur in real-world systems. In the paper we show how incorporating measures of UV absorbance and fluorescence, which relate to DOM fouling, with measures of PFAS provides a more complete picture of overall adsorption behavior in complex solution mixtures - and this allows accurate prediction of real-world system performance from rapid lab bench studies.
So we overcame decades-long challenges to obtain accurate simulations of real-world treatment systems from rapid benchtop experiments, and provided a cheap and field-ready way to monitor treatment performance for removal of really-difficult-to-treat water pollutants of serious emerging concern. Friggin awesome y’all.
Next steps for me are (besides working on the other papers I’m pushing though the peer review pipeline) to carve this work up into understandable and applicable bits for inclusion in different sections of the book project. That’s gonna take a minute. For now, I can offer up a couple of things to readers of this newsletter.
One thing, for paying subscribers, is that I’ve made an attempt at a Cliff’s Notes version of this just-published study, which incidentally is one of the hardest papers I’ve ever worked on. In the post I go through several of the key figures from the paper to provide succinct interpretations of the most salient, important, and novel contributions to adsorption science and engineering made by this study. The prose is qualitative, but the equations are included with the figures and data so you plug-and-chug number crunchers out there are free to go to town. This should be arriving in paying subscribers’ inboxes shortly.
For everybody on the list, I want to float an admittedly squishy concept that I’ve come to as a result of working on this study in trying to explain the overall adsorption behavior of chemical contaminants of concern when treating real-world waters (i.e., waters containing background dissolved organic matter or DOM for short): population-level adsorption behavior.
To be honest I’m not sure this is very much of an altogether new concept. I don’t remember seeing the term anywhere before, so maybe the term itself is new. “Population-level adsorption behavior” is just what coalesced in my head during the many discussions that co-author Kyle Shimabuku and I had over the months last year while trying to understand, and explain, what the data were telling us. I came back to this term again in several instances when writing the Cliff’s Notes version of the paper some of you will be getting shortly.
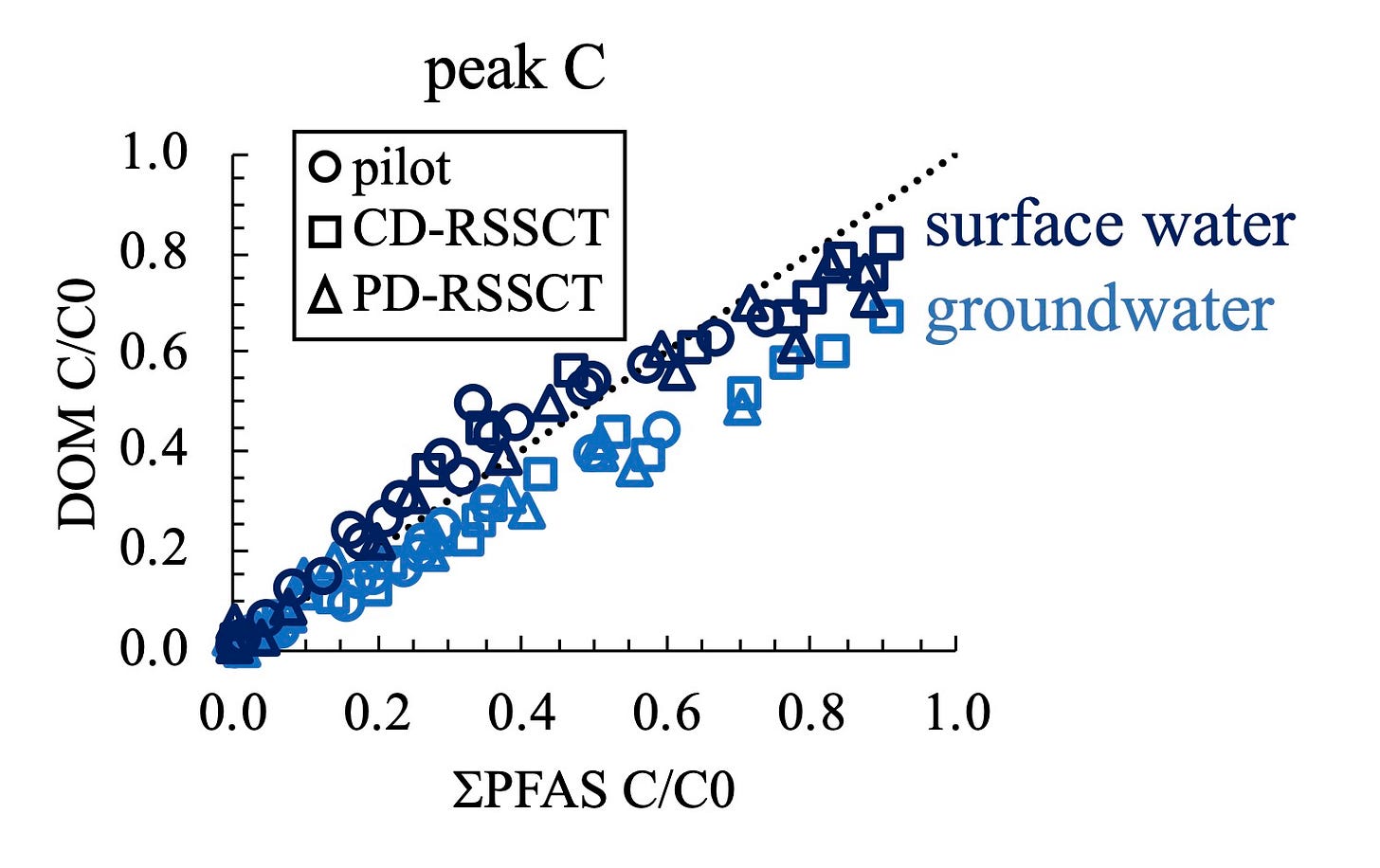
I cherry-picked some data from the paper to make the plot below and illustrate the point:
What you are looking at is a plot of the breakthrough of DOM (y-axis) compared with the breakthrough of PFAS (x-axis). In this case, it’s not all components of DOM, but specifically the components of DOM that absorb UV light and fluoresce in the “peak C” region. Don’t worry about the details, just understand that “peak C” tends to be components of DOM that like to adsorb on carbon surfaces (i.e., are “adsorbable”), and are comparatively small in molecular weight. “Small” in this case means similar in size to most target organic chemical pollutants such as herbicide molecules, rather than large bio-polymers like proteins and humic substances. Being adsorbable and similar in size to target pollutant molecules makes peak C DOM molecules good contenders to compete for adsorption sites within the carbon pore structure.
Housekeeping: Breakthrough of peak C DOM and PFAS are shown as fractions ranging from 0.0 to 1.0 corresponding to effluent concentration divided by influent concentration, instead of absolute concentrations. And, PFAS are shown as the sum of all (11) test compounds (denoted sigma-PFAS).
Kyle and I spent a lot of time starting at plots like this trying to figure out what we were seeing. What was weird to us was the way the three data series for each water type collapsed so neatly into respective trends. (Sure, there is a little scatter in the two data series, but if you’ve done wet chemistry research you’ll recognize that these trends appear remarkably clean and composed.)
I mentioned above and previously that one of the major contributions of this paper to adsorption research is that we found a way to reliably and accurately scale rapid small scale column data to predict full-scale target compound removal. For decades researchers have struggled to get CD- and PD-RSSCT data to match up to full-scale performance, and have not been super successful. The main problem is that DOM fouling behaves differently at the small column scale than at the large column scale.
It’s natural that the emphasis in column studies is placed on scaling of individual target pollutants - these are what we are concerned about removing from water after all. But thinking just of target pollutants alone abstracts them from their context - swirling around in a soup of similar and dissimilar molecules of DOM, most all of which are unknown.
Based on what we know about the difficulty of achieving accurate scaling of target pollutants in the presence of DOM, the CD-RSSCT, PD-RSSCT, and pilot column data series should not have lined up they way they so neatly did. Kyle and I stared at versions of plots like the one above and thought, “Wait - did that work? It wasn't supposed to work. Is it a coincidence? Or is something going on here?” (“Double rainbow man! What does this mean?”)
We apparently were observing consistency in the relationship between DOM adsorption and PFAS adsorption at different column scales - something that’s not supposed to happen according to the past few decades of research on RSSCTs. How to explain that?
The answer, we think, is that plotting the data this way provides a window (albeit an imperfect window) into, say it with me, population level adsorption behavior.
Not only were we treating PFAS as a group of roughly similar adsorbates by summing their breakthrough, we were treating the sub-population of DOM molecules most likely to compete with PFAS for adsorption sites on a group basis by tracking breakthrough of fluorescence peak C. Here’s how we explained it in the paper:
“There are conceptual reasons why treating mixtures of PFAS (or other OMPs) as a group of competitive adsorbates (i.e., using the sum- mation approach to breakthrough, ƩPFAS) could improve scaling and prediction by RSSCTs over individual-adsorbate approaches. Here it is illustrative to think of the constituents of competitive DOM and the mixture of individual PFAS as constituting an overall population of molecules with overlapping ranges of adsorbabilities, competing with one-another for adsorption sites. UV and fluorescence parameters do not detect individual DOM molecules but rather UV-active molecular features present among the population of competitive DOM molecules. There is thus a purposeful analogy for treating PFAS not on an individual basis but as a group of compounds with similar adsorption proper- ties to their UV-active DOM counterparts. The analogy is not perfect – e.g., constituents of non-competitive DOM can also be UV-active, and moieties of competitive DOM can be UV-inert.”
This phenomenon, if true, is what underpins the two main breakthrough contributions of the paper - a method to achieve accurate scaling of target pollutant removal using rapid lab bench experiments, and a cheap(-er) and field-ready(-ish) surrogate method for monitoring treatment system performance for target compound removal without having to quantify individual pollutants themselves (which is very sophisticated and expensive).
There’s a saying in Permaculture that “the problem is the solution.” Of course, a lot of Permaculture is hippy nonsense [;>)]. But in this case, we found a way to make the problem be the solution - the problem being DOM fouling, and the solution being to figure out how to track DOM foulants using comparatively cheap and easy methods using optical spectroscopy as a proxy for adsorbent bed life and side step the need for mass spec which for cost and logistical reasons isn’t feasible in a lot of situations.
Population level adsorption behavior, man. A more holistic approach to adsorption. Anytime I can take a more holistic approach to something it warms my hippy little heart.
In semi-related news I got to be a guest recently on the Doomer Optimism podcast. We had a really fun discussion that ranged over biochar and water, science and academia (and para-academia), “Global Sustainable Development” and other scams foisted on the world by professional managerial class technocrats, and “the one answer to all scientific questions.” Give it a listen!

...sounds like fine work for application where it counts most!
Hey Josh, congratulations on the new paper (won't even try to pretend that I understand what you're talking about regarding it!). Was happily surprised to see you on the DO substack, didn't realise it also had a podcast, checking it out now. Keep up the good work!